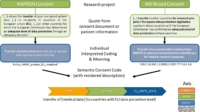
There are a number of challenges to overcome in the field of consent-based medical research. Due to the large number of national and international research initiatives, the content and structure of consent documents are usually project-specific. The documentation and semantic annotation of this consent information also generally follow different existing technical and semantic approaches. Furthermore, the simple and standardized retrievability of consent information poses a practical problem.
The challenge is essentially to find a suitable compromise for the technical implementation of patient consent that enables simple and uniform retrieval of consent information (consent query), even if the necessary input information is very complex and heterogeneous.
For this reason, we have developed the Semantic Consent Code (SCC). This consists of semantic axes (CLASS, ACTION, PURPOSE, ACTOR) and enables the description of consent documents regardless of their structure and form. The Semantic Consent Code thus creates a flexible and standardized prerequisite for the processing of consent data and at the same time supports the practical application of patient consent to research data. The convertibility of existing consent codes from Consent Policies to SCC (and vice versa) has already been successfully evaluated.
The new publication on “Semantic Consent Code (SCC)” provides further insights. This publication was published Open Access in July 2024 and can be cited as:
Translated with DeepL.com (free version)
Bialke M*, Hampf C, Blumentritt A, Moser FM, Lang S, Stehn A Sargsyan E, Hoffmann W, Kraus M. #consented – a semantic consent code to facilitate consistent documentation and implementation of consent in collaborative medical research. INTERNATIONAL JOURNAL OF MEDICAL INFORMATICS. (open access) 7/2024; 190(105545). DOI:10.1016/j.ijmedinf.2024.105545
Enjoy reading, sharing, and referencing.