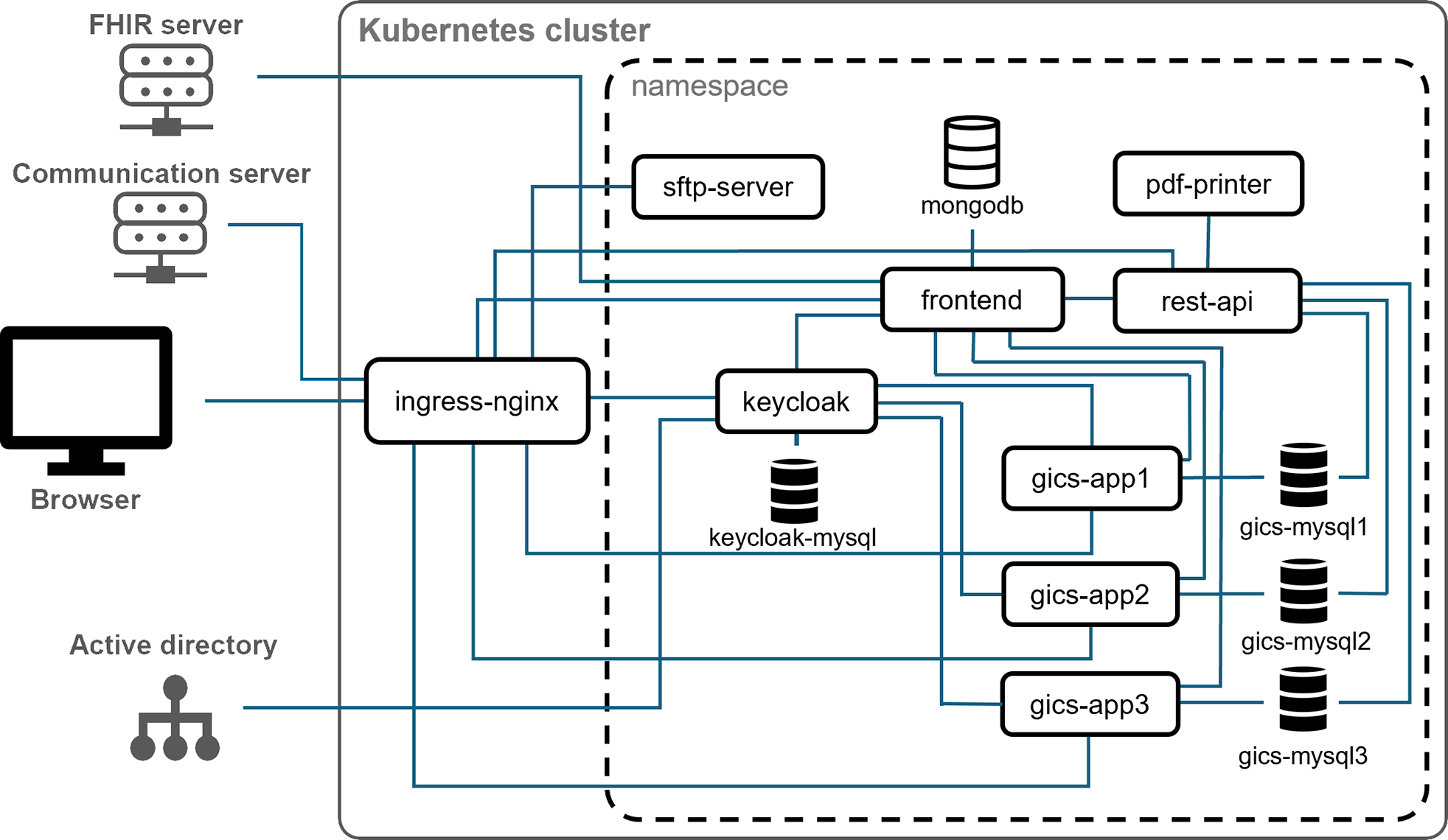
Participation in clinical trials requires informed consent. This is particularly important when storing samples in biobanks for future research. In pediatric studies, consent must be obtained from both the child and the legal representatives, which requires multiple consents to be recorded simultaneously. Electronic consents have become increasingly popular due to their error avoidance and the simplification of documenting multiple consents. However, the integration of these electronic consents into existing study software remains a challenge.
Under the direction of Dr. Danhauser, the aim of the work at the Ludwig-Maximilians-University Munich (Department of Pediatrics and Pediatric Polyclinic) was to validate the suitability of the generic Informed Consent Service (gICS) of the University Medicine Greifswald (UMG) for obtaining electronic consent in pediatric studies. The study was conducted in a pediatric research context, with additional consent obtained separately for the biobank. Over a period of 54 weeks, 1061 children and adolescents aged 3 to 17 years participated in the study, 348 of whom also agreed to participate in the biobank.
A total of 2066 consents and assents were analyzed, of which 945 were paper-based and 1121 electronic consents. The study showed a significant 94.7% reduction in the error rate of electronic consents. These results provide valuable insights into the use of gICS in various studies and the practical implementation of electronic consent software in pediatric medicine.
The publication was published Open Access in November 2024 and can be cited as follows Danhauser K, Mantoan LDL, Dittmer JM, Leutner S, Endres S, Strniscak K, et al. (2024) On-site electronic consent in pediatrics using generic Informed Consent Service (gICS): creating a specialized setup and collecting consent data. PLOS Digit Health 3(11): e0000661. https://doi.org/10.1371/journal.pdig.0000661
Enjoy reading, sharing and referencing.