The collection, processing and use of medical research data usually requires an informed consent of the person concerned, the so-called Informed Consent (IC) (see Art. 6-11 EU-GDPR). In the context of constantly growing national and international research initiatives, a reliable and efficient procedure for the digital administration of consents and revocations is necessary.
The collection, processing and use of medical research data usually requires an informed consent of the person concerned, the so-called Informed Consent (IC) (see Art. 6-11 EU-GDPR). In the context of constantly growing national and international research initiatives, a reliable and efficient procedure for the digital administration of consents and revocations is necessary.
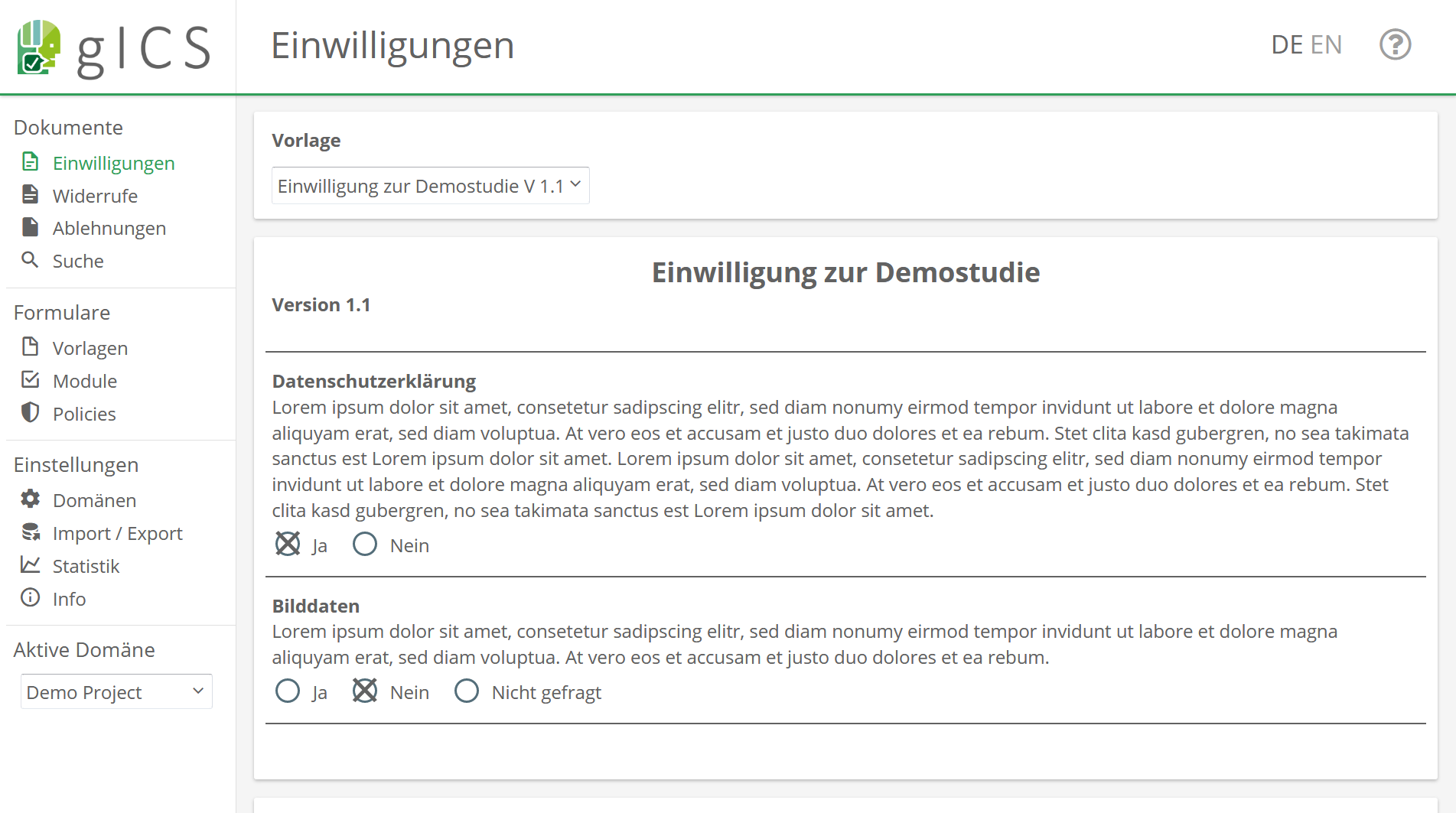
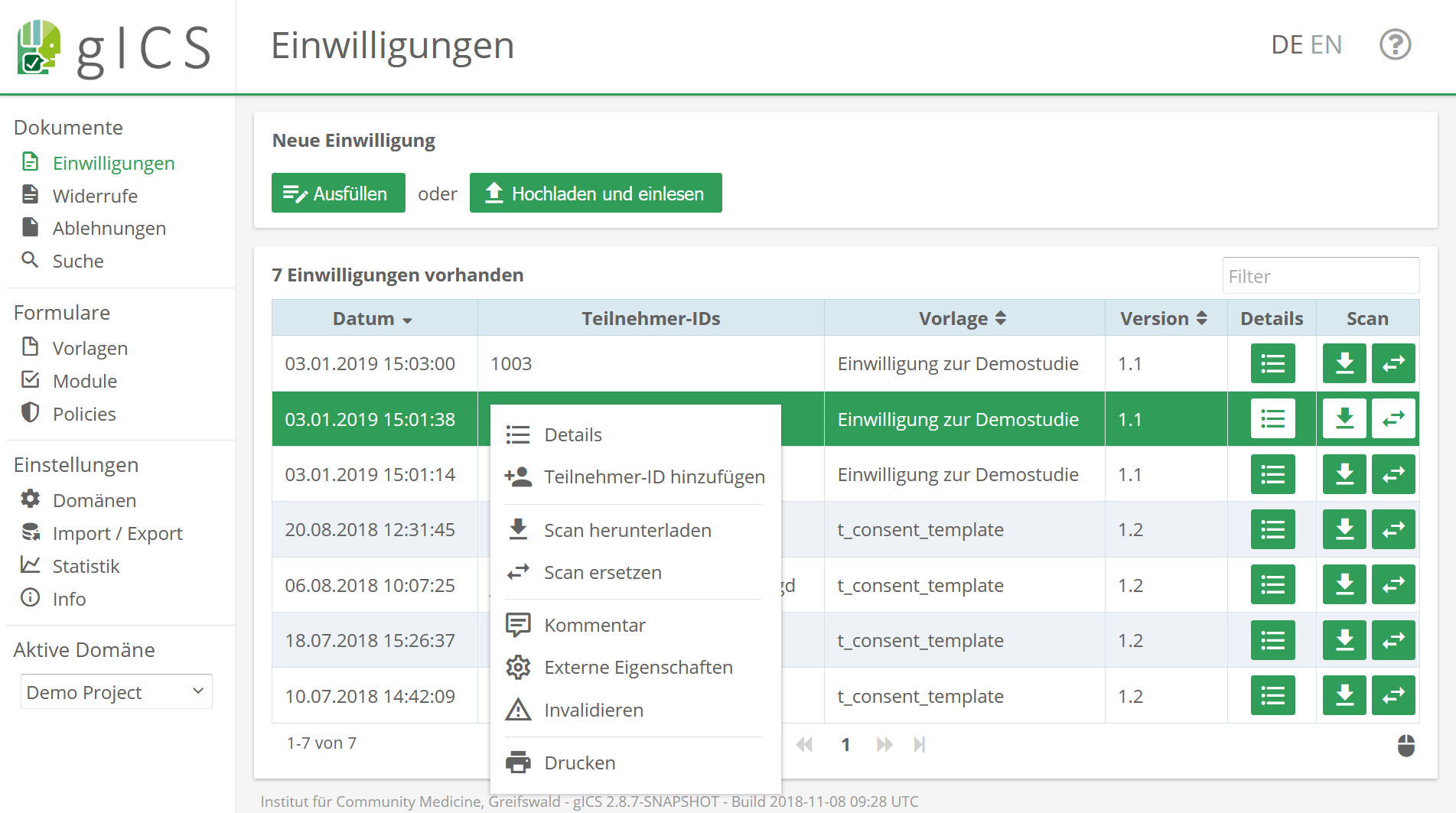
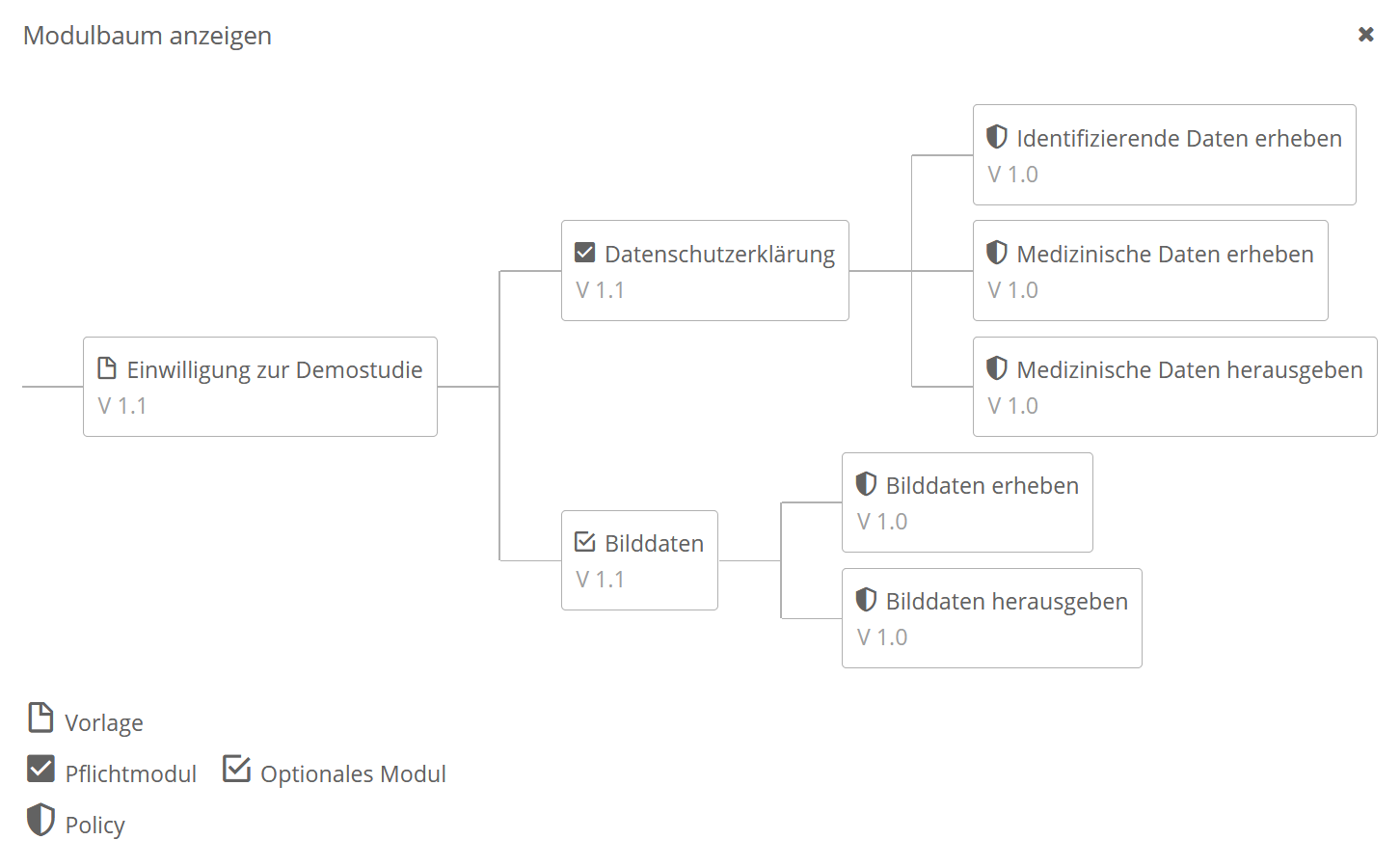
The gICS was developed for this purpose by the Trusted Third Party Greifswald. It can be integrated into both paper-based and purely digital workflows, and is used to process consent and revocation in the study context. In practice, it’s a good idea to combine single content- or logically related process steps of data processing (policies like collecting, transferring and storing data) into modules (e.g. module “Data Handling”). In gICS, templates represent an uncompleted but structured consent document. It contains modules that can be agreed to or declined by the participant as well as additional information of the consent (answer options, mandatory modules, free texts, etc.).
Who uses the gICS?
The diagram shows productive (green) and planned projects (light green).
Date: March 2025
Based on the feedback known to us
You are interested in our gICS? You can either try it out online in our demo or install it via Docker.